This paper is a bit afield from the usual material I cover but this is an interesting reaction. Shoji and coworkers have prepared the two-coordinate boron species 1,1 and confirmed its geometry by an x-ray crystal structure. What I find interesting is its reaction with CO2, which gives 2 and organoboranes that are not identified, though presumably derived from 3.
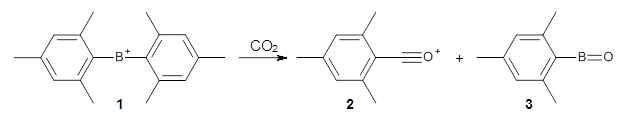
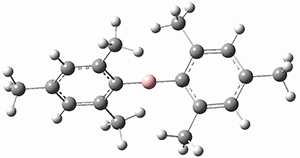
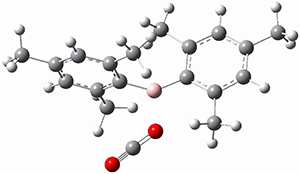
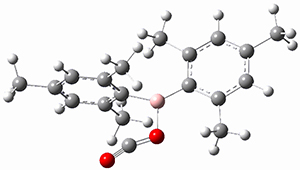
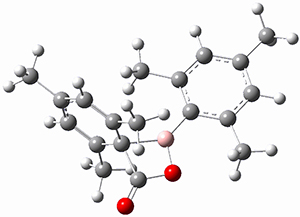
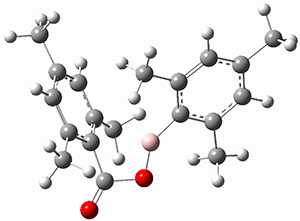
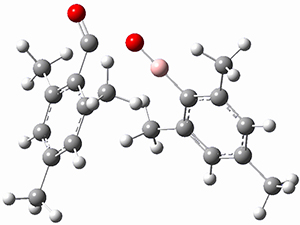
M06-2x/6-311+G(d,p) computations support a hypothetical mechanism whereby first a complex between 1 and CO2 is formed (CP1), that is 4.4 kcal mol-1 above isolated reactants. Then passing through TS1, which is 4.2 kcal mol-1 above CP1, an intermediate is formed (INT), which is almost 6 kcal mol-1 below starting materials. A second transition state is then traversed (about 1 kcal mol-1 below starting materials), to form an exit complex between 2 and 3, which can then separate to the final products with an overall exothermicity of 10.6 kcal mol-1. The structures of these critical points are shown in Figure 1.
|
1 |
CP1 |
|
TS1 |
INT |
|
TS2 |
CP2 |
Figure 1. M06-2x/6-311+G(d,p) optimized structures. Relative energy in kcal mol-1.
References
(1) Shoji, Y.; Tanaka, N.; Mikami, K.; Uchiyama, M.; Fukushima, T. "A two-coordinate boron cation featuring C–B+–C bonding," Nat. Chem. 2014, 6, 498-503, DOI: 10.1038/nchem.1948.
InChIs
1: InChI=1S/C18H22B/c1-11-7-13(3)17(14(4)8-11)19-18-15(5)9-12(2)10-16(18)6/h7-10H,1-6H3/q+1
InChIKey=WLUJABFTLHAEMI-UHFFFAOYSA-N
2: InChI=1S/C10H11O/c1-7-4-8(2)10(6-11)9(3)5-7/h4-5H,1-3H3/q+1
InChIKey=CUJVTHUIQVMVHD-UHFFFAOYSA-N
3: InChI=1S/C9H11BO/c1-6-4-7(2)9(10-11)8(3)5-6/h4-5H,1-3H3
InChIKey=ZJKBFARYTPYYGV-UHFFFAOYSA-N

Henry Rzepa responded on 08 Aug 2014 at 10:02 am #
INT caught my attention. It is a most unusual Wheland intermediate, in which this intermediate is actually more stable than the starting materials. Not very common I fancy. Nor, come to that, is carbon dioxide acting as an electrophile in an aromatic electrophilic substitution! By the way, the calculations were actually done with a solvent field (not mentioned above); for such ionic species, that is pretty much essential.
So now we can add this to the text book examples of introductory organic chemistry.