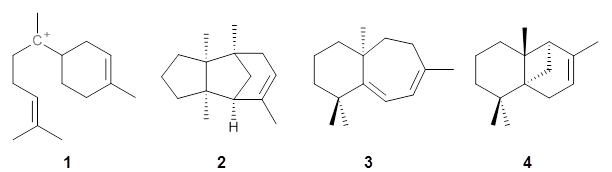
Hong and Tantillo1 report a real tour de force computational study of multiple pathways along the routes towards synthesis of a variety of sesquiterpenes. The starting point is the bisabolyl cation 1, and a variety of rearrangements, cyclizations, proton and hydride transfers are examined to convert it into such disparate products as barbatene 2, widdradiene 3, and champinene 4. The pathways are explored at mPW1PW91/6-31+G(d,p)//B3LYP/6-31+G(d,p). Some new pathways are proposed but the main points are the sheer complexity of the C15H25+ potential energy surface and the interconnections between potential intermediates.
References
(1) Hong, Y. J.; Tantillo, D. J. "Branching Out from the Bisabolyl Cation. Unifying Mechanistic Pathways to Barbatene, Bazzanene, Chamigrene, Chamipinene, Cumacrene, Cuprenene, Dunniene, Isobazzanene, Iso-γ-bisabolene, Isochamigrene, Laurene, Microbiotene, Sesquithujene, Sesquisabinene, Thujopsene, Trichodiene, and Widdradiene Sesquiterpenes," J. Am. Chem. Soc. 2014, 136, 2450-2463, DOI: 10.1021/ja4106489.
InChIs
1: InChI=1S/C15H25/c1-12(2)6-5-7-14(4)15-10-8-13(3)9-11-15/h6,8,15H,5,7,9-11H2,1-4H3/q+1
InChIKey=YKHXORRQMGBNFI-UHFFFAOYSA-N
2: InChI=1S/C15H24/c1-11-6-9-13(2)10-12(11)14(3)7-5-8-15(13,14)4/h6,12H,5,7-10H2,1-4H3/t12-,13-,14+,15-/m0/s1
InChIKey=RMKQBFUAKZOVPQ-XQLPTFJDSA-N
3: InChI=1S/C15H24/c1-12-6-7-13-14(2,3)9-5-10-15(13,4)11-8-12/h6-7H,5,8-11H2,1-4H3/t15-/m0/s1
InChIKey=SJUIWFYSWDVOEQ-HNNXBMFYSA-N
4: InChI=1S/C15H24/c1-11-6-9-15-10-12(11)14(15,4)8-5-7-13(15,2)3/h6,12H,5,7-10H2,1-4H3/t12-,14-,15-/m1/s1
InChIKey=XRDHEPAYTVHOPC-BPLDGKMQSA-N

Henry Rzepa responded on 14 Mar 2014 at 12:12 am #
Steve, No coordinates extracted to put into Jmol on your blog? The transition states are always interesting. I know it sometimes sounds like a broken record, but it is worth restating; data matters!
In the UK, the funding councils have a policy of “best practice in research data management, or RDM”, and from 2015 all UK-research council funded projects will have to demonstrate as part of their deliverables that they have properly managed their data.
If anyone is going to the ACS Dallas, there will be a whole day session Monday on this theme!
Steven Bachrach responded on 14 Mar 2014 at 10:47 am #
To be fair to Dean Tantillo and his student, they did deposit the coordinates of the structures they computed in the supporting materials (see http://pubs.acs.org/doi/suppl/10.1021/ja4106489). I did not include any structures in my post because to me the main point was the scope of the PES examined, not any specific transformation. So don’t hold mydecision against Dean!
Data does matter – I think the ethos of this blog reflects that sentiment. I include structures when it helps to understand the points I am raising in my posts, which are designed mostly to highlight the new features of the paper and inspire my readers to seek out the cited papers for more details.