One of the great challenges to computational chemistry and computational biochemistry is rational design of enzymes. Baker and Houk have been pursuing this goal and in their recent paper they report progress towards an enzyme designed to catalyze a Diels-Alder reaction.1
They envisaged an enzyme that could catalyze the Diels-Alder of 1 with 2 by having a suitable hydrogen bond acceptor of the carbamide proton of 1 (such as the carbonyl oxygen of glutamine or asparagine) along with a suitable donor to the oxygen of 2 (such as the hydroxyl of tyrosine, serine or threonine) – as shown below. Along with positioning the diene and dienophile near each other and properly orienting them for reaction, the activation barrier should be lowered by narrowing the HOMO-LUMO gap.
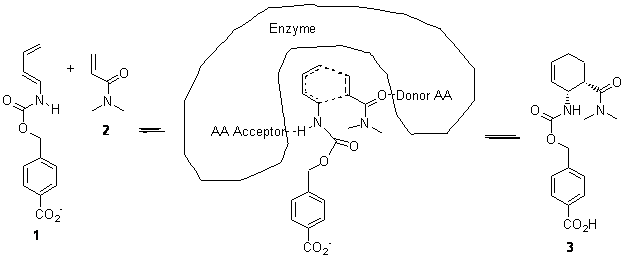
A series of transition states for the Diels-Alder reaction of 1 with 2 along with the hydrogen-bonded amino acids were optimized B3LYP/6-31+G(d,p) and used as constraints within the RosettaMatch code for locating a protein scaffold that could accommodate this TS structure. This resulted in 84 protein designs, each of which were synthesized and screened for activity in catalyzing the Diels-Alder reaction. Of these potential enzymes, 50 were soluble and of these 50, only 2 showed any activity. These two were selectively mutated to try to improve activity, and some improvement was obtained.
Of particular note is that mutation that removed one or both of the residues designed to hydrogen bond to the substrates resulted in complete loss of activity.
In principle 8 different steriosomeric products are possible in the reaction of 1 with 2. In solution in the absence of enzyme, four products are observed, with the major product (47%) the 3R,4S endo prodcut 3. The designed enzymes were constructed to make this product, and in fact it is the only observed stereoisomer formed in the reaction in the presence of enzyme. Furthermore, the designed enzymes are quite selective; for example, changing a single N-methyl group to N-ethyl on 2 reduced the rate by a factor of 2 and larger substituents resulted in a greater rate suppression.
Turnover rate is high and suggests that these enzymes might have real application in chemical synthesis. The disappointing aspect of the study was the poor ratio of predicted enzymes (84) to ones that actually had activity (2).
References
(1) Siegel, J. B.; Zanghellini, A.; Lovick, H. M.; Kiss, G.; Lambert, A. R.; St.Clair, J. L.; Gallaher, J. L.; Hilvert, D.; Gelb, M. H.; Stoddard, B. L.; Houk, K. N.; Michael, F. E.; Baker, D., "Computational Design of an Enzyme Catalyst for a Stereoselective Bimolecular Diels-Alder Reaction," Science, 2010, 329, 309-313, DOI: 10.1126/science.1190239

Eugene responded on 18 Jan 2012 at 1:13 pm #
The year in that paper is actually 2010, not 2011.
Steven Bachrach responded on 18 Jan 2012 at 2:42 pm #
Thanks Eugene – I have corrected this. Just goes to show how backlogged my queue is!