The click reaction, the copper-assisted cycloaddition of an azide with an alkyne, has been extended to biological systems by use of a strained alkyne (cyclooctyne) thereby eliminating the need of the toxic copper agent.1 Fox has extended this analogy with the reaction of strained trans-cyclooctene 1 with tetrazine 2.2
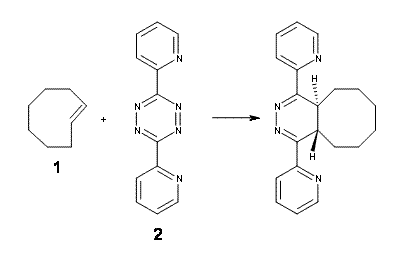
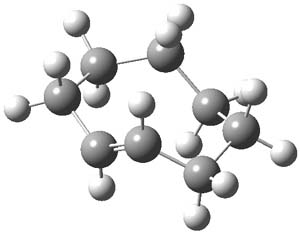
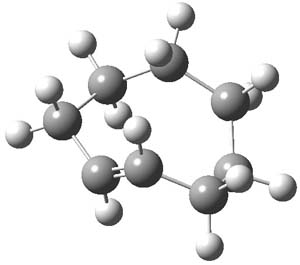
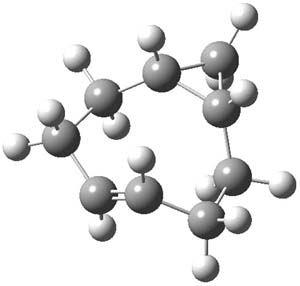
The interesting new twist here is to add more strain to trans-cyclooctene to perhaps make the cycloaddition even faster. Bach3 had pointed out that the half chair conformation of 1 is almost 6 kcal mol-1 higher in energy than the ground state (Figure 1). Fox suggests that fusing a cyclopropyl ring to the eight-member ring would create a ring in the half chair 3. Since 3 would be even more strained than 1, it should undergo a faster cycloaddition reaction.
|
1 |
1 (half chair) |
|
3 |
|
Though Fox did not estimate the strain of 3, I have computed the structure of 1 constrained to the geometry of 3, with the two hydrogens that replace the bonds to the cyclopropyl carbon allowed to optimize. This restricted geometry is in fact 6.1 kcal mol-1 (M06L/6-311+G(d,p)) higher in energy than 1 – so the fusion of the 3-member ring does net the strain increase expected by Bach.
Fox reports estimates of the free energy of activation (at M06L/6-311+G(d,p)) for the reaction of 1or 3 with 2. The barrier for the raction with trans-cyclooctene 1 is 8.92 kcal mol-1, while the barrier for the reaction with 3 is 6.95 kcal mol-1. A methylenehydroxyl derivative of 3 was synthesized and it does react 180 times faster than the reaction with 1. Furthermore, the differences in the experimental free energies of activation is 3.0 kcal mol-1, in excellent agreement with the computed difference.
References
(1) Agard, N. J.; Prescher, J. A.; Bertozzi, C. R., "A Strain-Promoted [3 + 2] Azide-Alkyne Cycloaddition for Covalent Modification of Biomolecules in Living Systems," J. Am. Chem. Soc., 2004, 126, 15046-15047, DOI: 10.1021/ja044996f
(2) Taylor, M. T.; Blackman, M. L.; Dmitrenko, O.; Fox, J. M., "Design and Synthesis of Highly Reactive Dienophiles for the Tetrazine-trans-Cyclooctene Ligation," J. Am. Chem. Soc., 2011, 133, 9646-9649, DOI: 10.1021/ja201844c
(3) Bach, R. D., "Ring Strain Energy in the Cyclooctyl System. The Effect of Strain Energy on [3 + 2] Cycloaddition Reactions with Azides," J. Am. Chem. Soc., 2009, 131, 5233-5243, DOI: 10.1021/ja8094137
InChIs
1: InChI=1/C8H14/c1-2-4-6-8-7-5-3-1/h1-2HInChIKey
2: InChI=1/C12H8N6/c1-3-7-13-9(5-1)11-15-17-12(18-16-11)10-6-2-4-8-14-10/h1-8H
InChIKey=JFBIRMIEJBPDTQ-UHFFFAOYAE
3: InChI=1/C9H14/c1-2-4-6-9-7-8(9)5-3-1/h1-2,8-9H,3-7H2/b2-1+/t8-,9+
InChIKey=YWIJRSGCJZLJNV-YLSDFIPEBO