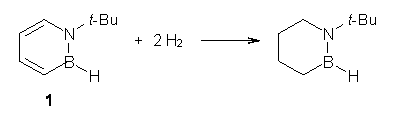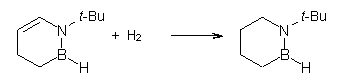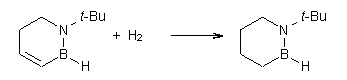1,2-Azaborine appears to be aromatic (see my previous post). Can the extent of aromatic character be measured? Well, obviously the first thing one must decide is just which “aromaticity metric” to choose. Dixon and Liu have now measured the aromatic resonance stablilization energy (ASE) through computations and heats of hydrogenation.1
One can set up to hydrogenation comparisons. First, obtain the hydrogenation of 1,2-azaborine itself. They used the t-butyl analog 1, so the hydrogenation is given in Reaction 1.
|
|
Reaction 1 |
Then as comparison, one can perform two separate hydrogenations, looking at the double bond adjacent to the nitrogen (Reaction 2) and the double bond adjacent to the boron (Reaction 3).
|
|
Reaction 2 |
|
|
Reaction 3 |
The heat of hydrogenation of Reaction 1 is -30 ± 1 kcal mol-1 (-30.1 at G3(MP2). The heats of hydrogenations of reactions 2 and 3 are -22.7 ± 0.5 kcal mol-1 (-23.8) and -23.9 ± 0.7 (-24.7), respectively. The difference between the sum of reactions 2 and 3 and Reaction 1 is the ASE: 16.6 kcal mol-1 (18.4 at G3(MP2)). This can be compared to the ASE of benzene determined in the analogous way to be 32.4 kcal mol-1. Therefore, 1,2-azoborine is aromatic, but appreciably less so than benzene, which is consistent with the NICS computations (see the post).
References
(1) Campbell, P. G.; Abbey, E. R.; Neiner, D.; Grant, D. J.; Dixon, D. A.; Liu, S.-Y., "Resonance Stabilization Energy of 1,2-Azaborines: A Quantitative Experimental Study by Reaction Calorimetry," J. Am. Chem. Soc., 2010, 132, 18048-18050, DOI: 10.1021/ja109596m