The most favorable conformation of a carboxylic acid is the Z form. In fact, the E form is rarely found. Schreiner now offers an explanation for why this is so.1
Photolysis of matrix-deposited benzoic acid revealed only the Z form (1Z). However, photolysis of deuterated benzoic acid did reveal the E form 1E, however it disappeared with a half-life of 12 minutes on argon at 11 K and 20 K. The lack of temperature dependence, and the huge isotope effect suggested that the isomerization proceeds via tunneling.
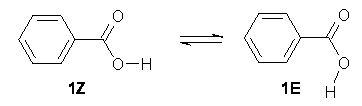
The tunneling rate was computed by generating the reaction path at CCSD(T)/cc-pVTZ with
MP2/cc-pVDZ zero point energy. This gave a half-life of 2.8 h for the deuterium species and 10-5 min for the proton species. A Hammet-like relationship could be produced for the half-lives of para-substituted benzoic acids. Interestingly, a nice correlation is found between the computed width of the tunneling barrier and the half life with σ-donating ability.
References
(1) Amiri, S.; Reisenauer, H. P.; Schreiner, P. R., "Electronic Effects on Atom Tunneling: Conformational Isomerization of Monomeric Para-Substituted Benzoic Acid Derivatives," J. Am. Chem. Soc., 2010, 132 , 15902–15904, DOI: 10.1021/ja107531y
InChIs
Benzoic acid: InChI=1/C7H6O2/c8-7(9)6-4-2-1-3-5-6/h1-5H,(H,8,9)/f/h8H
InChIKey=WPYMKLBDIGXBTP-FZOZFQFYCI

Henry Rzepa responded on 05 Feb 2011 at 9:14 am #
It would be interesting to see if the NBO interaction terms for the OH lone pair also correlate with the Hammett values. Also, the equivalent of a hydrogen with a mass of 4 (DOI: 10.1126/science.1199421 has recently been made. Presumably the half life with this isotope will be even longer!
Rob Paton responded on 06 Feb 2011 at 8:30 am #
A comment on the opening remark here…
Esters, for which tunneling is (presumably) a great deal less important, also adopt the Z form preferentially. So whether conformational isomerization occurs via tunneling or rotation about the C-O single bond (feasible at 298K), I would argue that the position of the E/Z equilibrium is still best understood in terms of minimization of dipole-dipole repulsion and maximization of O donation into the CO sigma*.
Well, that’s what I teach undergraduates anyway…
Rob
Computational Organic Chemistry » Domino Tunneling responded on 11 Aug 2015 at 8:14 am #
[…] between its conformations,1 despite observation of tunneling in other carboxylic acids (see this post). This was rationalized by computations which suggested rather high rearrangement barriers. […]