Here’s a nice example of the productive interplay between experiment and computations.1 The dipeptide N-Acyl-Ala-Ala-Benzyl was prepared and subjected to UV and IR/UV analysis. The IR showed two separate structures with distinctly different environments for the NH bonds: one structure showed intramolecular hydrogen bonding while the other did not.
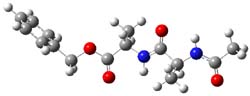
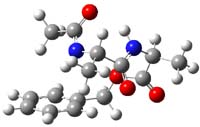
B97/TZVPP computations revealed two structures. The first is a linear dipeptide with intramolecular hydrogen bonding occurring in a 5,5 relationship. (There are actually three conformers of this but all have similar energy, only one is shown in Figure 1.) The second structure displays a bent shape with a NH-π interaction, also shown in Figure 1. The computed vibrational spectra for each structure matches up well with the NH region of the experimental IR.
Figure 1. B97-D/TZVPP optimized structures of N-Acyl-Ala-Ala-Benzyl.
The authors spend a great deal of time noting that the 0 K energies predict that the second structure, being 4 kcal mol-1 more stable, should be the only one observed. However, since the jet cooling will likely trap the structures at their 300 K distribution, this could account for the existence of two structures. However, when the computations include entropy corrections, so now we’re looking at ΔG(200 K), B97-D and MO6-2x suggest that the two structures are very close in energy. But they caution that MP2 predicts a large energy gap unless atomic counterpoise corrections are used to account for intramolecular basis set superposition (see this post), a problem that appears to be much less severe with the DFT methods.
References
(1) Gloaguen, E.; de Courcy, B.; Piquemal, J. P.; Pilme, J.; Parisel, O.; Pollet, R.; Biswal, H. S.; Piuzzi, F.; Tardivel, B.; Broquier, M.; Mons, M. J. Am. Chem. Soc, 2010, 132, 11860-11863, DOI: 10.1021/ja103996q
InChIs
InChI=1/C15H20N2O4/c1-10(16-12(3)18)14(19)17-11(2)15(20)21-9-13-7-5-4-6-8-13/h4-8,10-11H,9H2,1-3H3,(H,16,18)(H,17,19)/t10-,11-/m0/s1/f/h16-17H
InChIKey=KRIKKPGWLXOEAS-VFIKCTIADD

Henry Rzepa responded on 17 Oct 2010 at 8:23 am #
I am surprised that the original article does not mention, never mind address, the interesting observations that optical rotation can be used in reverse as a method for conformational exploration. One presumes that this dipeptide was made using enantiomerically pure amino acids, in which case the optical rotations must be available. The connection between this measurement and conformation has been made many times.