Often gem-dialkyl substitution accelerates a reaction, for example in the formation of an epoxide via reaction 1. Here the relative rates are 1:21:252 in going from 1 to 2 to 3.1 This acceleration is the Thorpe-Ingold effect and had been suggested to arise from a steric reaction: that the methyl groups contract the angle and bring the terminal groups closer together.
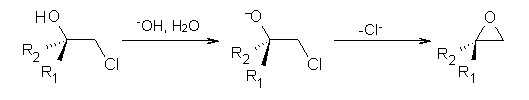
1: R1 = R2 = H
2: R1 = Me, R2 = H
3: R1 = R2 = Me
Kostal and Jorgensen2 have examined the reaction of the 2-chloroethoxides 1-3 using computations, especially to look at the effect of solvent. At MP2/6-311+G(d,p) and CBS-Q, the relative rates (based on the activation free energy ΔG‡) are 1:2.8:17 and 1:0.7:3.7, respectively. Evidently there is no significant rate enhancement afforded by gem-substitution in the gas phase.
However, solution computations give a very different result. Using PCM along with the MP2 method, the computed relative rates are 1:5.8:1100 and with the Monte Carlo-Free Energy Perturbation method, the relative rates for aqueous solution are 1:30:773. Thus, the Thorpe-Ingold acceleration is due to solvent. Analysis of the hydrogen bonded structures and the solute-water pair distributions suggest that increasing alkyl substitution reduces the strength of solvation of the reactant, leading to the lower activation barrier.
References
(1) Jung, M. E.; Piizzi, G., "gem-Disubstituent Effect:Theoretical Basis and Synthetic Applications," Chem. Rev., 2005, 105, 1735-1766, DOI: 10.1021/cr940337h
(2) Kostal, J.; Jorgensen, W. L., "Thorpe-Ingold Acceleration of Oxirane Formation Is Mostly a Solvent Effect," J. Am. Chem. Soc., 2010, 132, 8766-8773, DOI: 10.1021/ja1023755

Henry Rzepa responded on 28 Jul 2010 at 12:36 am #
There are probably many interesting steric effects lurking in the literature. Another one that was famous in its time, but is rather less well remembered now is Newman’s (he of the projection) Rule of six (DOI: 10.1021/ja01166a123 formulated in 1950. A more recent application of this rule in 1989 was to the properties of tocopherol (DOI: 10.1021/jo00264a012, one of the authors of which, Ingold, is the same as of the Thorpe-Ingold effect). The rule was stated by the latter as “the greater the number of atoms in the six position the greater will be the steric effect”, i.e., the importance of 1,6 steric interactions increases as the number of atoms connected by five bonds (the “six number”) increases”
Since modelling nowadays can handle both solvation and dispersion interactions, we can look forward to more of these unexplained effects noted in the past receiving analysis.
Henry Rzepa responded on 02 Aug 2010 at 10:28 am #
A colleague mentioned to me today that the Thorpe-Ingold effect is much utilized in radical (cyclization) chemistry (DOI: 10.1139/v83-190 and 10.1021/ja042595u). It seems very unlikely that solvation would be the principle controlling influence in such reactions. Perhaps the ionic example cited in this blog is really an exception to the Thorpe-Ingold?